If you are looking for BBCCT-109 IGNOU Solved Assignment solution for the subject Metabolism of Carbohydrates and Lipids, you have come to the right place. BBCCT-109 solution on this page applies to 2021-22 session students studying in BSCBCH courses of IGNOU.
BBCCT-109 Solved Assignment Solution by Gyaniversity
Assignment Code: BBCCT-109 / TMA / 2021 - 2022
Course Code: BBCCT-109
Assignment Name: Metabolism of carbohydrates and lipids Core Course in Biochemistry
Year: 2021 -2022
Verification Status: Verified by Professor
Note: Attempt all questions. The marks for each question are indicated against it.
Write the answers in your own words; do not copy from the course material.
PART-(A)
Marks: 50
Q1. Define the terms: (5X2= 10)
Q1. (a) Chemoautotrophs
Ans) Chemoautotrophs are creatures that get their energy from a chemical reaction (chemotrophs), but their carbon source is carbon dioxide, which is the most oxidised form of carbon (CO2). Chemoautotrophs make all of the organic molecules they need from carbon dioxide.
Q1. (b) Anaplerotic reactions
Ans) Anaplerotic reactions are anabolic processes that aid in the generation of biochemical pathway intermediate molecules. Anaplerotic pathways are the intermediate reaction steps in such reactions. Anaplerotic reactions are a crucial element of metabolism, as they are involved in metabolic pathways such as citric acid metabolism and lipid production.
Q1. (c) Obligate anaerobes
Ans) Obligate anaerobes are microorganisms that are killed by typical oxygen concentrations in the atmosphere (20.95 percent O2). Oxygen tolerance varies by species, with some species thriving in settings with up to 8% oxygen and others losing viability in situations with more than 0.5 percent oxygen.
Q1. (d) Fermentation
Ans) Fermentation is a metabolic process that uses enzymes to cause chemical changes in organic substrates. It is described as the extraction of energy from carbohydrates in the absence of oxygen in biochemistry.
Q1. (e) Catabolism
Ans) Catabolism is a group of metabolic pathways that break down molecules into smaller units, which are then oxidised for energy or employed in other anabolic reactions. Large molecules are broken down into smaller pieces through catabolism. The breaking-down aspect of metabolism is catabolism, and the building-up aspect is anabolism.
Q 2. (a) Which factors account for high phosphoryl group transfer potential of ATP? Name two compounds having phosphoryl group transfer potential higher than ATP. (4+1)
Ans) The structural similarities between ATP and its hydrolysis products explain why the products are more stable than ATP. The hydrolysis of ATP releases a significant amount of energy. In the triphosphate unit, energy is preserved. Electrostatic repulsion, product resonance stability, and hydration-induced stabilisation all contribute to ATP's strong phosphoryl group transfer potential. Among the high-energy molecules found in biological systems, ATP is in the middle. This enables it to operate as an effective phosphoryl group carrier. It can take phosphate from substances like PEP and use it to regenerate ATP and transfer it to other cells with lesser transfer potential. This could be one of the reasons it was chosen as the universal carrier of free energy.
In biological systems, some molecules have a higher phosphoryl transfer potential than ATP. Phosphoenolpyruvate (PEP), 1,3-bisphosphoglycerate (1,3-BPG), and creatine phosphate are examples of these molecules.
Q2. (b) What is glycolysis? Draw reactions of its two phases and label their reactants, products and enzymes. (5)
Ans) Glycolysis is a set of chemical events that aid in the extraction of energy from glucose. This is an old metabolic pathway that can still be found in the majority of living species today. Both aerobic and anaerobic cellular respiration are based on it. It occurs in a cell's cytosol and is the basis for both aerobic and anaerobic cellular respiration.
In the cytoplasm, the entire reaction of glycolysis is represented simply as:
C6H12O6 + 2 NAD+ + 2 ADP + 2 P —–> 2 pyruvic acid, (CH3(C=O)COOH + 2 ATP + 2 NADH + 2 H+
Step 1: Hexokinase
The first step in glycolysis is the conversion of D-glucose into glucose-6-phosphate. The enzyme that catalyzes this reaction is hexokinase.
Here, the glucose ring is phosphorylated. Phosphorylation is the process of adding a phosphate group to a molecule derived from ATP. As a result, at this point in glycolysis, 1 molecule of ATP has been consumed.
Step 2: Phosphoglucose Isomerase
The second reaction of glycolysis is the rearrangement of glucose 6-phosphate (G6P) into fructose 6-phosphate (F6P) by glucose phosphate isomerase (Phosphoglucose Isomerase).
The second step of glycolysis involves the conversion of glucose-6-phosphate to fructose-6-phosphate (F6P). This reaction occurs with the help of the enzyme phosphoglucose isomerase (PI). As the name of the enzyme suggests, this reaction involves an isomerization reaction.
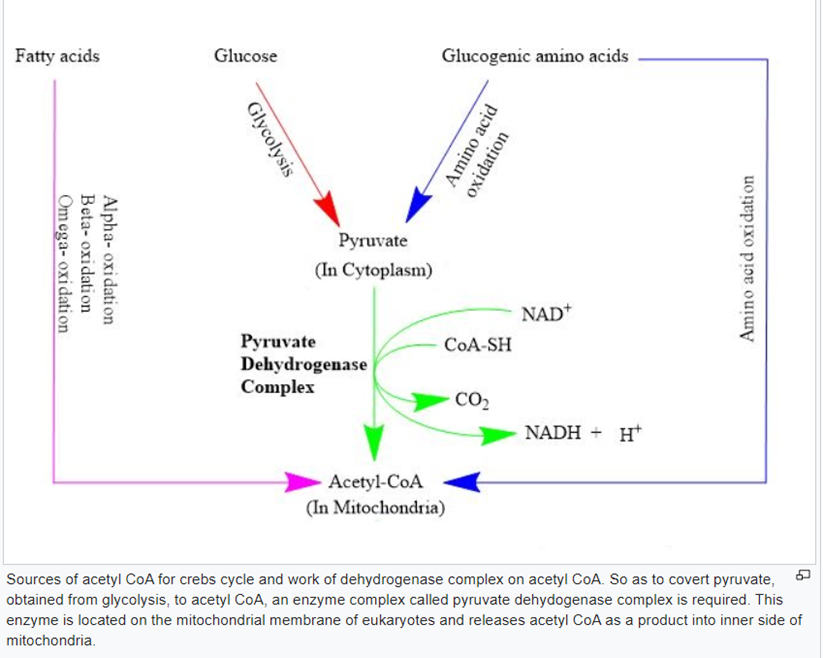
Q3. (a) With the help of a neatly labeled diagram, explain different steps of conversion of pyruvate to acetyl CoA by PDH complex. (5)
Ans) Metabolism, like many other bodily activities, is not a simple mechanism. Metabolism contains a lot of complicated steps, even though it is done quite quickly. The process is catalysed by several enzymes that catalyse the various steps. Taking pyruvate (from glycolysis) and converting it to CO2 and acetyl-CoA is one of the metabolic processes (used in the krebs cycle). An enzyme is required in order to carry out this action in a quick and efficient manner. Pyruvate dehydrogenase is the enzyme that catalyses this reaction.
Pyruvate dehydrogenase is an extremely complicated enzyme that catalyses the intricate steps of metabolism. The pyruvate dehydrogenase enzyme complex is made up of three different enzymes: E1, E2, and E3. These enzymes are found several times throughout the structure since it has a complicated structure. Pyruvate dyhydrogenase is a dehydrogenase in general. Pyruvate dehydrogenase, like most dehydrogenases, works on NAD+ as a cofactor. NAD+ accomplishes its goal by converting NAD+ to NADH+H+. This enzyme complex achieves the reaction without releasing its substrate into the reaction solution, which is known as substrate channelling. TPP, Lipoate, and FAD are the names of the cofactors associated to the enzyme.
Q3. (b) Write a short note on Pentose phosphate pathway and its importance. (5)
Ans) The pentose phosphate route is a glucose metabolism alternative to glycolysis. It's also known as the phosphogluconate route or the hexose monophosphate shunt (HMP shunt). The discovery of an alternative pathway was predicated on the discovery that traditional glycolysis inhibitors had no effect on glucose use in specific tissues. It was also discovered that glucose labelled in C-1 oxidises more quickly than glucose labelled in C-6.
Importance of Pentose Phosphate Pathway
The pentose phosphate pathway (PPP) is a branch of the glycolytic or gluconeogenic pathway that produces NADPH and ribose 5-phosphate (R5P) and shunts carbons back to the glycolytic or gluconeogenic pathway. The PPP has been shown to be a key regulator of cellular homeostasis and biosynthesis in terms of reduction-oxidation (redox). Many human diseases are thought to be aided by enzymes found in the PPP. The role of the PPP in type 2 diabetes and cancer will be discussed in this review.
Q4. (a) Justify, glycolysis and gluconeogenesis from pyruvate are not simple reversal of each other. (5)
Ans) In glycolysis, glucose is converted into pyruvate; in gluconeogenesis, pyruvate is converted into glucose. However, gluconeogenesis is not a reversal of glycolysis. Several reactions must differ because the equilibrium of glycolysis lies far on the side of pyruvate formation. The actual ΔG for the formation of pyruvate from glucose is about -20 kcal mol-1 (-84 kJ mol-1) under typical cellular conditions. Most of the decrease in free energy in glycolysis takes place in the three essentially irreversible steps catalyzed by hexokinase, phosphofructokinase, and pyruvate kinase.
In gluconeogenesis, the following new steps bypass these virtually irreversible reactions of glycolysis:
Phosphoenolpyruvate is formed from pyruvate by way of oxaloacetate through the action of pyruvate carboxylase and phosphoenolpyruvate carboxykinase.
2. Fructose 6-phosphate is formed from fructose 1,6-bisphosphate by hydrolysis of the phosphate ester at carbon 1. Fructose 1,6-bisphosphatase catalyzes this exergonic hydrolysis.
3. Glucose is formed by hydrolysis of glucose 6-phosphate in a reaction catalyzed by glucose 6-phosphatase.
Q4. (b) Explain coordinated regulation of glycogenesis and glycogenolysis. (5)
Ans) Within a cell, gluconeogenesis and glycolysis are coordinated so that one route is relatively inactive while the other is quite active. If both sets of reactions were extremely active at the same time, a total of four nucleotide triphosphates (two ATP and two GTP) would be hydrolyzed per reaction cycle. Under cellular conditions, both glycolysis and gluconeogenesis are extremely exergonic, therefore there is no thermodynamic barrier to such simultaneous action. However, the numbers and activity of each pathway's specific enzymes are regulated so that neither pathway is overly active at the same moment. The rate of glycolysis is influenced by glucose concentrations, while the pace of gluconeogenesis is influenced by lactate and other glucose precursors.
Fructose 6-phosphate and fructose 1,6-bisphosphate interconversion is strictly regulated. Phosphofructokinase is stimulated by AMP, whereas it is inhibited by ATP and citrate. Citrate activates fructose 1,6-bisphosphatase, which is inhibited by AMP and activated by citrate. The presence of a high quantity of AMP indicates that the energy charge is low and that ATP production is required. High quantities of ATP and citrate, on the other hand, suggest a high energy charge and an abundance of biosynthetic intermediates. Glycolysis is practically shut off and gluconeogenesis is boosted in these settings.
Q5. (a) What is photorespiration? Explain its significance? (5)
Ans) Photorespiration (also known as C2 photosynthesis or the oxidative photosynthetic carbon cycle) is a process in plant metabolism in which the enzyme RuBisCO oxygenates RuBP, losing some of the energy gained by photosynthesis. The desired reaction is the addition of carbon dioxide to RuBP (carboxylation), which is a crucial step in the Calvin–Benson cycle. However, about 25% of RuBisCO reactions add oxygen to RuBP (oxygenation), resulting in a product that cannot be used in the Calvin–Benson cycle. This mechanism affects photosynthesis efficiency, potentially lowering photosynthetic production by 25% in C3 plants. The exchange of metabolites between chloroplasts, leaf peroxisomes, and mitochondria occurs during photorespiration, which is a complicated network of enzyme processes.
Significance of photorespiration:
Photorespiration aids in the disposal of energy when stomata close due to water stress throughout the day.
By dispersing excess excitation energy, photorespiration protects the plant from photoxidative damage.
Q5. (b) Explain the partitioning of fixed carbon to sucrose and starch synthesis in leaf cells. (5)
Ans) The ultimate end products of photosynthetic carbon dioxide uptake by most leaves are sucrose and starch. The two processes are separated; sucrose is produced in the cytosol while starch is produced in the chloroplasts. Sucrose (the primary carbohydrate) is transferred from the source (leaves) to the sink (non-photosynthetic tissues) in the majority of plants during active photosynthesis, and surplus fixed carbon is temporarily converted to starch and stored in the chloroplasts.
The regulatory mechanisms match the rates of carbon fixation with the partitioning of fixed carbon into sucrose and starch. The effects of 3-PGA and inorganic phosphate on the rate of ADP-G production in spinach leaves were discovered in the late 1960s. It was later discovered to be a generic mechanism in all plants investigated. 3-PGA is an activator and Pi is an inhibitor of ADP-G pyrophosphorylase, which is the glucose donor for starch production. During light-dark transitions, the concentration of these metabolites fluctuates in the chloroplast; low [Pi/ [PGA] ratio in light. The pace of starch synthesis during the day has been linked to the variation in their ratio in intact leaf systems.
A Pi/ triose phosphate translocator transports the triose phosphates (3-PGA and DHAP) across the chloroplast membranes and returns Pi in exchange for triose phosphate. The photosynthetic rate and Pi release during sucrose synthesis determine the rate of exchange. 3-PGA suppresses the synthesis of the modulator fructose 2,6 bisphosphate in the cytosol, causing fructose 1,6 bisphosphatase and sucrose synthesis to be stimulated. In the presence of Pi, the opposite occurs. The kinase responsible for fructose 2,6 bisphosphate production in plant leaves is not phosphorylated. Changes in triose phosphate concentrations, as well as oscillations in fructose 2,6 bisphosphate concentrations, provide a feed forward mechanism that allows sucrose synthesis to be coordinated with carbon fixation rate. Because Pi is low in the chloroplast and fructose 2,6 bisphosphate is abundant in the cytosol, as sucrose accumulates in the leaf, an increasing proportion of fixed carbon is transferred to starch production.
Finally, sucrose synthesis is controlled at the sucrose synthase level. Glucose 6-phosphate activates it, while Pi inhibits it. Because of the exchange for triose phosphates, Pi is low during active sucrose production. Photosynthesis ceases at night, and stored starch is eaten. As a result, the amount of starch in the chloroplast decreases at night and is converted to sucrose for export.
PART- (B)
Marks: 50
Q6. (a) What are the end products of β- oxidation of odd and even chain fatty acids? Describe regulation of β- oxidation of fatty acids? (1+4)
Ans) Beta-oxidation is primarily facilitated by the mitochondrial trifunctional protein, an enzyme complex associated with the inner mitochondrial membrane, although very long chain fatty acids are oxidized in peroxisomes.
The overall reaction for one cycle of beta oxidation is:
End Products of Odd Chain Fatty Acid Oxidation Fatty acid is : Acetyl-CoA(C2): Enter in TCA cycle and get completely oxidized. Propionyl-CoA(C3): Is Converted into Succinyl CoA (4C), A TCA intermediate.
The only difference is the final product that is produced. In the case of even chain fatty acids, we generate acetyl CoA molecules. But in the case of odd chain fatty acids, we generate acetyl CoA as well as a propionyl CoA molecule.
Regulation of Beta-oxidation of Fatty Acids
Lipolysis and β-Oxidation of Fatty acids are well regulated under Hormonal influence.
Insulin secretion occurs in well fed condition that inhibits Lipolysis of Fat (TAG) and mobilization of Free Fatty acids. Therefore, insulin decreases β Oxidation of Fatty acids.
Glucagon and Epinephrine secretion occurs in Emergency Condition: When Cellular or Blood Glucose lowers down there is secretion of these hormones. Glucagon and Epinephrine stimulates Lipolysis in emergency condition.
Glucagon and Epinephrine stimulates the Enzyme Hormone sensitive Lipase and hydrolyzes Fat (TAG). These hormones helps to mobilize Free fatty acids out into blood circulation and Increases β Oxidation of Fatty acids.
Q6. (b) Define ketogenesis, is it a normal, physiological process? Explain why it goes up in conditions of starvation and uncontrolled diabetes? (5)
Ans) Ketogenesis is the biochemical process through which organisms produce ketone bodies by breaking down fatty acids and ketogenic amino acids. The process supplies energy to certain organs, particularly the brain, heart and skeletal muscle, under specific scenarios including fasting, caloric restriction, sleep, or others. In rare metabolic diseases, insufficient gluconeogenesis can cause excessive ketogenesis and hypoglycemia, which may lead to the life-threatening condition known as non-diabetic ketoacidosis.
The amounts of ketones produced in diabetic patients are much higher than those observed in fasting subjects, as diabetic patients show decreased insulin levels (impaired insulin secretion or ineffective insulin action), increased levels of counterregulatory hormones such as glucagon that increase free fatty acid
In diabetic ketoacidosis (DKA), high levels of ketones are produced in response to low insulin levels and high levels of counterregulatory hormones. In acute DKA, the ketone body ratio (3HB:AcAc) rises from normal (1:1) to as high as 10:1. In response to insulin therapy, 3HB levels commonly decrease long before AcAc levels. In normal individuals the presence of insulin counter regulates the effects of glucagon leading to the inhibition of HSL as well as that of CPT-1; as a result, very little ketogenesis occurs due to limited fatty acid availability. However, in diabetic patients with insulin deficiency, especially as seen in T1D, all of these pathways are upregulated by counter regulatory hormones, in most part by glucagon. In the absence of insulin the inhibitory effect on HSL and CPT-1 are lost leading to the increased influx of FFA and production of ketones in diabetic patients.
Q7. (a) Calculate the number of ATP produced during conversion of palmitate to acetoacetate in liver with justification. (5)
Ans) Theoretically ATP yield for every oxidation cycle can be maximum upto 17, as NADH produces 3 ATP, FADH2 = 2 and end product, acetyl COA governed Citric Acid Cycle produces 12 ATP. Cumulatively in practice it comes 14 ATP for a one time full oxidation cycle and it only possible when we assume, 2.5 ATP per NADH molecule and 1.5 for each FADH2 molecule and 10 fro citric acid cycle, which seems to be irrational. But for better understanding we put this kind of assumption. However palmitate is 16 carbon containing saturated fatty acid.
So, for an even-numbered saturated fat (C2n) ("C" indicating the number of carbon atoms and noted 2n is in the subscript), n - 1 oxidations are required, and the final process yields an 1 more acetyl CoA i.e. for palmitate if 2n=16 then n=8, which required n-1 oxiation i.e 7, so there will be 7 FAD, 7 NADH and 8 acetyl COA which will produce 1.5 ATP, 2.5 ATP and 10 ATP respectively and cumulatively it will be calculated 108 where 2 ATP were used in the initial activation of fatty acid so 2 ATP will be subtracted and total number of ATP will become 106. But if you go with the thereotical yields and have larger production ATP source then values of NADH, FAD and ATP produced by the full rotation of citric acid cycle will produce 3, 2, 12 ATPs. Together ATP number will become 131 and 2 will be subtracted as required for initial activation of fatty acid so you will remain with 129.
Q7. (b) Illustrate organization of various domains of animal fatty acids synthase and write their activities. (5)
Ans) The synthesis of saturated fatty acid is catalysed by fatty acid synthase (FAS) complex which brings about the reductive synthesis of fatty acids in steps of two. Its seven catalytic units are organised either as domains of a multifunctional enzyme (FAS 1) or separate polypeptides (FAS II). The basic chemistry of fatty acid synthesis is conserved through evolution. FAS-I is present in vertebrates and fungi. The animal FAS are a homodimer of 260kd subunits (Fig. 10.3).Each chain is folded into three domains; domain 1 is substrate entry and condensation unit, domain 2 is reduction unit and domain 3 is the product release unit. The two identical chains are arranged anti-parallel such that domain I of each chain of the dimer interacts with domain 2 and 3 of the other chain. Although multiple enzymes are present on same chain in FAS I, their reactions are spatially isolated and the flexible arm of ACP carries the intermediates from one active site to another. Thus two functional units consist of domains from different chains. In this system only the final product (palmitate) synthesised after multiple cycles is released.
Figure shows Organisation of animal fatty acid synthase.
In FAS II each step is catalysed by a separate enzyme. The intermediates are released and then diffuse to the next enzyme; they may also be diverted to other routes. The FAS II generally ends up producing a variety of fatty acids. It is present in plants, bacteria and vertebrate mitochondria.
Q8. (a) Describe regulation of de novo lipogenesis in mammals. (5)
Ans) De novo lipogenesis is the process of synthesis of fatty acids starting from simple precursor (acetyl CoA). It is energy consuming and reductive process. The fatty acid chain is elongated in steps of two and the 2 carbon units are derived from acetylCoA. The synthesis of fatty acids starting from simple precursor (acetyl CoA) is known as de novo lipogenesis. In animals (and yeast), it occurs primarily in the cytosol of various tissues such as liver, adipose (fat) tissue, central nervous system and lactating mammary glands.
The more active fatty acid elongation system is present in ER of plants, mammals, yeast and other lower eukaryotes. It essentially works on the same theme as de novo lipogenesis. The system requires acyl- CoAs as primers, malonyl CoA as donor of 2C units and NADPH as reductant. The substrates are saturated and unsaturated acyl-CoA of chain length C16 or more. The basic reactions are condensation, reduction, dehydration and reduction. It plays an important role in the elongation of dietary essential fatty acids.
Q8. (b) Write steps in formation of stearic acid and oleic acid from palmitic acid. (5)
Ans) Palmitate (a C-16 fatty acid) is the major product of the fatty acid synthase complex. However, plants and animals also need fatty acids longer than C16 in their membranes and storage lipids. The elongation of fatty acids is catalysed by type III FAS or ‘elongases’ as they increase the chain length of a preformed fatty acid. These fatty acids may be either saturated or unsaturated. In many mammalian tissues, the elongation systems are present in the smooth endoplasmic reticulum and mitochondria. The elongation pathways use coenzyme-A as acyl carrier rather than acyl carrier protein (ACP) of fatty acid synthase complex and use NADPH instead of FADH2 for saturation of double bond.
The acetyl-CoA and malonyl-CoA are linked to the synthase and ACP, then there is a sequence of acetyl group transfers that runs a total of seven times to form palmitoyl-ACP, from which the palmitic acid is finally released. Palmitic acid is the precursor for variety of long-chain fatty acids such as stearic acid, palmitoleic acid, and oleic acid. Generally, there is either an elongation or sometimes a desaturation step. However, desaturation is a tricky process for vertebrates. The desaturation at C9 to form oleic acid from stearic acid can occur.
Q9. (a) Write in brief about the four stages of cholesterol biosynthesis. (5)
Ans) Cholesterol biosynthesis is a part of a branched pathway. The isoprenoid intermediates can be diverted for the synthesis of sugar carrier dolichol, coenzyme Q or the side chain of heme-a. Additionally, these intermediates are used in protein modification such as prenylation of proteins.
The synthesis of cholesterol can be divided into four stages:
1. Condensation of three acetate units to form mevalonate;
2. Conversion of mevalonate to activated isoprene units;
3. Polymerization of six 5-carbon isoprene units to 30-carbon linear squalene; and
4. Cyclization of squalene to form the steroid nucleus which undergoes a series of changes involving oxidations, removal and migration of methyl groups to produce cholesterol.
Lanosterol is finally converted to cholesterol in a series of about 20 reactions catalysed by microsomal enzymes that include loss of three methyl groups, reduction of bond at the position 24 (25) and isomeris
ation of double bond at 8(9) to position 5 as shown in the figure below:
Cholesterol is a sterol characteristic of animal cells; plants, fungi, and protists make other, closely related sterols instead. They use the same biosynthetic pathway as far as squalene 2,3-epoxide, at which point the pathways diverge slightly, yielding other sterols, such as stigmasterol in many plants and ergosterol in fungi.
Q9. (b) How is cholesterol biosynthesis regulated? (5)
Ans) The amount of cholesterol that is synthesized in the liver is tightly regulated by dietary cholesterol levels. When dietary intake of cholesterol is high, synthesis is decreased and when dietary intake is low, synthesis is increased. However, cholesterol produced in other tissues is under no such feedback control. Cholesterol and similar oxysterols act as regulatory molecules to maintain healthy levels of cholesterol.
Sensitive Response Elements (SREs). SREs are found in the promoters of the genes coding for the enzymes of the cholesterol biosynthetic pathway and LDL receptors. Transcription factors important to activating SREs are Sterol Regulating Element Binding Proteins (SREBPs). Due to their ability to bind SREs, SREBPs play an instrumental role in cholesterol homeostasis. SREBPs serve to regulate all 12 enzymes in the cholesterol biosynthetic pathway including the rate limiting enzyme HMGCoA reductase (HMGR). High dietary sterol levels acting on SCAP ultimately stop the maturation of SREBPs, resulting in the down regulation of key enzymes such as HMGR, thus, reducing the amount of cholesterol produced by the liver. Limiting cholesterol synthesis leads to a homeostatic response in which cells increase the density of LDL receptors on their surfaces. This increases the clearance rate of LDL particles from the plasma and reduces plasma LDL cholesterol and its related health risks. The decrease in cholesterol synthesis also promotes an increase of HDL, thus, clearing even more cholesterol from the plasma.
Q10. (a) How is ceramide converted to phosphosphingolipids? (5)
Ans) The parent compound of all sphingolipids is ceramide (N-acyl sphingosine). It is synthesised in the endoplasmic reticulum. The biosynthesis of ceramide begins with the condensation of palmitoyl CoA with serine by a pyridoxal phosphate dependent 3-ketosphinganine synthase (serine palmitoyl transferase) forming 3-ketosphinganine (3-ketodihydrosphingosine). In step 2, 3-ketosphinganine is reduced to sphinganine (dihydrosphingosine). Nacylation of dihydrosphingosine by acyl CoA transferase yields dihydroceramide (N-acylsphinganine). Finally, desaturation of dihydroceramide forms ceramide.
Once the backbone is ready, ceramide can be converted either to phosphosphingolipids (sphingomyelin) or glycosphingolipids. Ceramide is also a signaling molecule.
Synthesis of Sphingomyelin from Ceramide
The head group attachment to ceramide occurs in the Golgi complex and to some extent in the plasma membrane. The donor of phosphocholine head group is phosphatidyl choline (PC) and not CDP-choline. The reaction is catalysed by sphingomyelin synthase. The polar head group (no net charge) is linked to the backbone by a phosphodiester linkage.
PC + Ceramide ------à Sphingomyelin +DAG
Q10. (b) What are different fates of glucose in liver in a well fed state? (5)
Ans) Generally excess glucose in liver and muscles is first converted to glucose 6-phosphate by glucokinase (in liver) or hexokinase (in muscles) and then to glucose 1-phosphate by phosphoglucomutase (PGM). In liver, gluconeogenesis can directly provide glucose 6-phosphate.
The reaction catalysed by UDP-glucose pyrophosphorylase proceeds in the direction of synthesis as the product pyrophosphate (PPi) is readily hydrolysed by a ubiquitous inorganic pyrophosphatase. The biosynthesis of many polymers including DNA and RNA are driven in one direction by hydrolysis of PPi.
The polymerizing enzyme glycogen synthase is incapable of initiating glycogen synthesis. It is a chain elongating enzyme. The initial steps of polymerisation are primed by a protein glycogenin. It is both an enzyme (glucosyl transferase) and a primer for assembling glycogen chains. The primer is synthesised by step by step addition of eight glucose residues from UDP-glucose by glucosyl transferase; the first one is attached to the tyrosine residue of glycogenin. Further elongation is taken over by glycogen synthase and glycogenin remains bound to the single reducing end.
Overall reaction:
(Glucose)n + Glucose + 2 ATP (Glucose)n+1 + 2ADP + 2 Pi
For each molecule of glucose incorporated into glycogen, two molecules of ATP are required. One is required for the phosphorylation of glucose and other for the conversion of UDP to UTP
100% Verified solved assignments from ₹ 40 written in our own words so that you get the best marks!
Don't have time to write your assignment neatly? Get it written by experts and get free home delivery
Get Guidebooks and Help books to pass your exams easily. Get home delivery or download instantly!
Download IGNOU's official study material combined into a single PDF file absolutely free!
Download latest Assignment Question Papers for free in PDF format at the click of a button!
Download Previous year Question Papers for reference and Exam Preparation for free!